FAES FARMA | DERIO facility
Developer and manufacturer of human medicinal products.

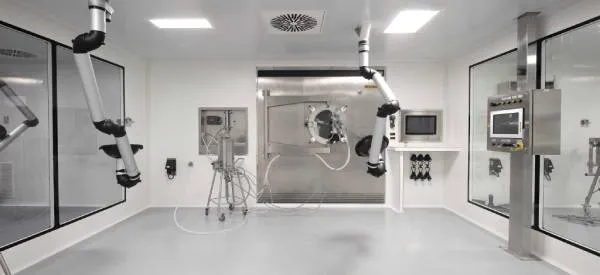











About the plant
Founded in 1933, FAES FARMA develops, manufactures and commercializes pharmaceutical products and APIs, with a wide-ranging international projection.
Today, FAES FARMA, the company that began more than 90 years ago in a small Basque factory, is now present in Europe, Latin America and Africa.
Indeed, our origins have defined us, but it is even more important to know how far we want to go: to take care of people s health today in order to have a healthier society tomorrow.
FAES FARMA CDMO
NICHE DRUG DOSAGE FORMS: UNIFILL (VERTICAL THERMOFORM FILL AND SEAL UNI-DOSE), SOFT GELATIN CAPSULES, BILAYER TABS & SOLID & LIQUID STICKS (UNI-DOSE).
ORAL SOLIDS (TABS, CAPS), LIQUIDS (SYRUPS, DROPS), TOPICALS, SUPPOSITORIES...
HIGH PHARMA QUALITY & CAPACITY
- Modern, high-capacity manufacturing plants.
- Development and manufacture of products marketed in more than 100 countries.
- GMPs: US FDA, ANVISA, Japan PMDA, European Agencies, etc.
COLLABORATION MODELS
Development
- Formulation design, optimization, and stability testing.
- Tailoring drug formulations for maximum efficacy in the development stage.
- GMP manufacturing for clinical trials.
- Small to medium batch sizes with a focus on flexibility and quality control.
Manufacturing
- Scalability assessment, process optimization, and pilot-scale production.
- Large-scale GMP manufacturing, up-scaling.
- Move to industrial scale with optimized processes and technology transfer support.
- Navigating the regulatory landscape with strategic support.
- Customized packaging solutions, distribution logistics, and compliance with global standards.
- Ensuring product integrity from development to global distribution.
Bulk & FDF
- Niche forms: Sticks, Bilayer tabs, Unifill, Soft Capsules.
- Traditional Forms: Tablets, ODT, Capsules, Suppositories, Ointments, Syrups, Drops...
Compliance
- ISO
- EMA (EU GMP)
- FDA (cGMP)
- Health Canada (Canada GMP)
- French Service-Public (CIR)
- ECOVADIS
- World Health Organization (GMP / HACCP)
- NMPA (China GMP)
- TGA (Australia GMP)
- ANVISA (Brazil B-GMP)
- Roszdravnadzor (Russia GMP)
- ANMAT (Argentina nueva GMP)
- MFDS/KFDA (Republic of Korea GMP)
- PMDA/MHLW (Japan GMP)
- ISP (Chile BPM)
- INVIMA
- MSPAS
- COFEPRIS
- DIGEMID
- MHRA (UK GMP)
Activity
- Oral liquids and semisolids, Oral solids / OSD, Sterile forms (ophtalmic, nasal, otic), Topical, mucosal and transdermal, Bulk, Sachets, Stick packs, Oral solutions, Oral suspensions, Oral emulsions, Syrups, Tablets, Hard capsules, SoftGel capsules, Granules / Pellets, Powders, Bottles, Dropper bottles, FDF / DRUG PRODUCTS manufacturing
Features
- Uses: Investigational, Commercial (Phase IV), Preclinical, Phase I, Phase II, Phase III, Human
- Toxicity (OEB classification): 1 / low-hazard (PDE > 5,000 µg/day)
- Controlled substance: N/A
- BSL: N/A
- Therapeutic areas: (A) Digestive tract and metabolism, (C) Cardiovascular system, (D) Dermatologicals, (G) Genito urinary system and sex hormones, (J) Antiinfectives for systemic use, (M) Musculoskeletal system, (N) Nervous system, (R) Respiratory system
- Markets: DIGEMID (Peru), COFEPRIS (Mexico), INVIMA (Colombia), MSPAS (Guatemala), ISP (Chile), FDA (USA), EMA (EU), PMDA (Japan), Health Canada (Canada), MHRA (UK), TGA (Australia), NMPA (China), EDE (UAE), ANVISA (Brazil), ANMAT (Argentina), MFDS (South Korea), Russian Health Authorities
Batch Size / Reactor
- Small, Medium, Large
Services
- Regulatory services, Development services, Manufacturing services, Analytical / QC services, Quality Assurance services, Packaging, Logistics, Preformulation studies, Clean room rental, R&D, Formulation / Galenic design, QbD (Quality by Design), Process development, Process optimization, Process validation, Stability studies design, Stability studies execution, ICH Stability studies, Tech transfer, Pilot plant, Formulation mixing, Research batches, Engineering batches, Scale-up, Pilot batches, Registration batches, Batch records, Release testing of raw materials, Release testing of product, Analytical methods transfer, Batch certification / release, GMP documentation, Primary packaging, Secondary packaging, Labeling, Serialization, Storage, Distribution, GDP, CMC regulatory support
Address
Parque Científico y Tecnológico de Bizkaia, Ibaizabal Bidea. Edificio 901 - 48160 DERIO (Vizcaya), SPAIN
Year
2024
 Spain
Spain
Documents
Related content
-
FDF CDMO Services | Finished Dosage Forms Contract Manufacturing
-
Leading Steriles CDMO Companies | Steriles Contract Manufacturing Services
-
Leading OSD CDMO Companies | Oral Solids Contract Manufacturing Services
-
Leading Oral Liquids CDMO Companies | Oral Liquid Formulations Contract Manufacturing Services
-
Leading Topical CDMO Companies | Transdermal Contract Manufacturing Services
Contact us
If you have any questions or suggestions, click here. We will be happy to assist you.






