Cambrex Charles City
Our 400+ team members in Charles City specialize in late-phase and commercial API development and cGMP manufacturing.





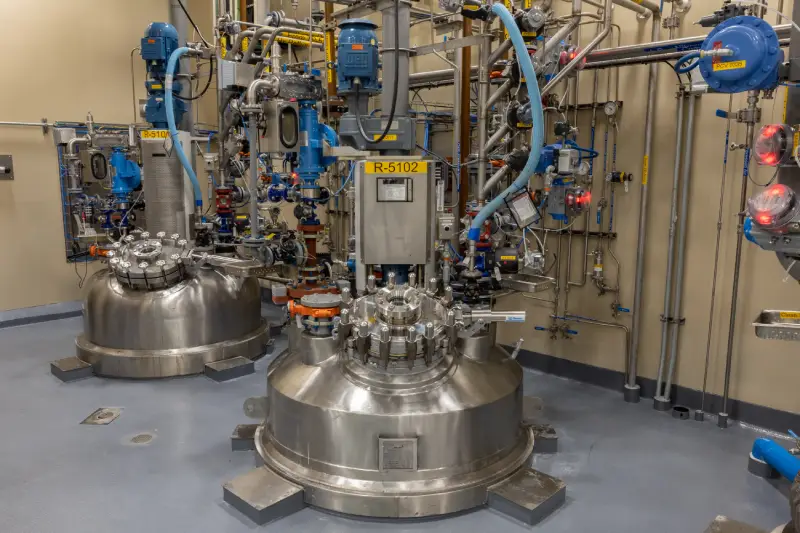




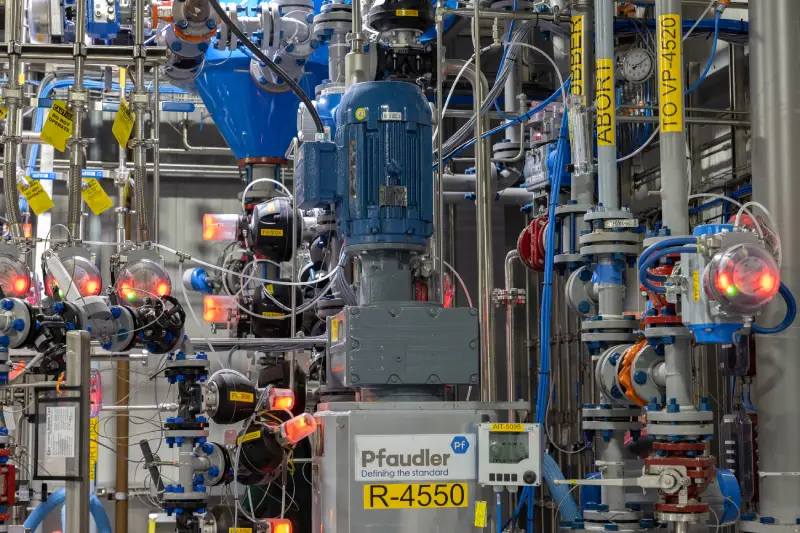

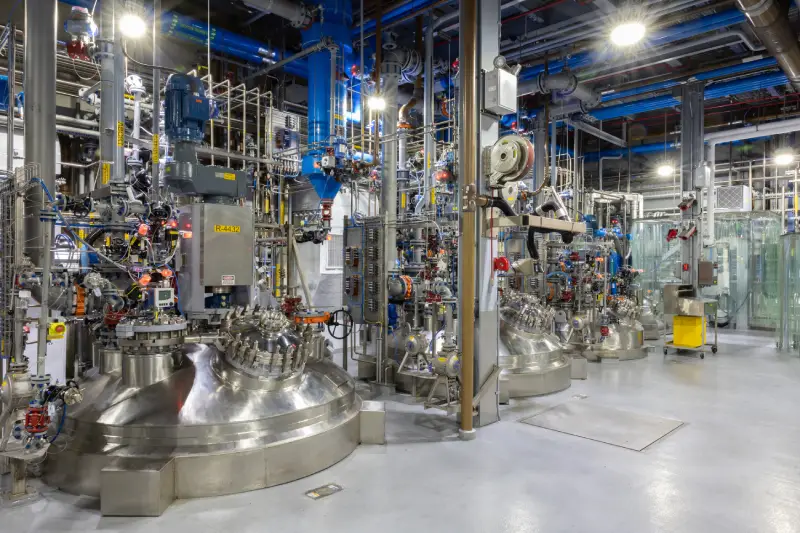



About the plant
Our 400+ team members in Charles City have strong expertise in custom development and cGMP commercial manufacturing. Located on a 45-acre campus, we manufacture a wide range of APIs and pharmaceutical intermediates, including highly potent molecules and controlled substances. Our facility is home to multiple commercial cGMP plants for large-scale production, while also offering resources for small- and mid-scale development and manufacturing. This Cambrex facility is one of a limited number to be authorized by the Drug Enforcement Administration to import narcotic raw materials on a commercial scale.
Facility Details
- 4 cGMP commercial-scale production plants
- 2 cGMP mid-scale plants
- 1 cGMP pilot plant
- 1 cGMP kilo laboratory
- 350,000 L commercial capacity
- R&D development and analytical laboratories
- Validated commercial continuous flow technology at large scale
- Controlled substance registrations to support all CS designations
- High potency development and manufacturing center
- Strong regulatory history
Certifications
- EMA (EU GMP)
- FDA (cGMP)
- Health Canada (Canada GMP)
- MFDS/KFDA (Republic of Korea GMP)
- PMDA/MHLW (Japan GMP)
- MHRA (UK GMP)
Activity
- CHEMICAL-SYNTHETIC, RSM (Raw Starting Materials), Intermediates, Fine Chemicals, API (Active Pharmaceutical Ingredients), Excipients, Building Blocks manufacturing
Features
- Uses: Investigational, Commercial (Phase IV), Preclinical, Phase I, Phase II, Phase III, Human
- Toxicity (OEB classification): 1 / low-hazard (PDE > 5,000 µg/day), 2 (PDE = 1,000 - 5,000 µg/day), 3 (PDE = 100 - 1,000 µg/day), 4 / HPAPI (PDE = 10 - 100 µg/day), 5 / HPAPI (PDE < 10 µg/day)
- Controlled substance: High potential for abuse & no medical use, High potential for abuse & medical use, Lower potential for abuse, Low potential for abuse, Lowest potential for abuse
- BSL: N/A
- Therapeutic areas: N/A
- Markets: FDA (USA), EMA (EU), PMDA (Japan), Health Canada (Canada), MHRA (UK), MFDS (South Korea)
Batch Size / Reactor
- Small, Medium, Large, 1 - 10 L, 10-100 L, 100 - 1,000 L, 1,000 - 2,000 L, 2,000 - 5,000 L, 5,000 - 10,000 L, > 10,000 L
Services
- Development services, Manufacturing services, Analytical / QC services, Quality Assurance services, Logistics, Preformulation studies, R&D, Formulation / Galenic design, QbD (Quality by Design), Process development, Process optimization, Process validation, Lyophilization cycle development, Stability studies design, Stability studies execution, ICH Stability studies, Comparability studies, Tech transfer, Pilot plant, Formulation mixing, Lyophilization, Research batches, Engineering batches, Scale-up, Pilot batches, Registration batches, Batch records, Analytical methods development, Analytical methods validation, Release testing of raw materials, Release testing of product, Analytical methods transfer, QP in house, Batch certification / release, GMP documentation, Storage, Distribution, GDP, Salt screening, Co-crystal screening, Polymorph screening, Crystallization screening, Chiral resolution screening, Milling, Safety studies, Solid form selection, Crystal engineering, PGI assessment (Genotoxic Impurities), Nitrosamine risk assessment, Customized block synthesis, Impurity synthesis
Address
1205 11th St Charles City, IA 50616, USA
Year
1927
 United States
United States
Other plants of this company
 Cambrex Waltham (Snapdragon Chemistry)
Cambrex Waltham (Snapdragon Chemistry)Our team members with Snapdragon Chemistry Inc., a Cambrex Company, specialize in chemical process development and manufacturing services, leveraging state-of-the-art technology to optimize your process and prepare scaled quantities of your product.
 Cambrex Agawam
Cambrex AgawamCambrex Agawam offers a full suite of microbiological, analytical, and environmental monitoring services to support pharmaceutical and medical device product development.
 Cambrex Waterford (Q1 Scientific)
Cambrex Waterford (Q1 Scientific)Q1 Scientific Ltd., a Cambrex Company, provides environmentally controlled cGMP stability storage services for the pharmaceutical, medical device, and life science industries.
Contact us
If you have any questions or suggestions, click here. We will be happy to assist you.





