Pharmaron API Commercial Synthesis Shaoxing (China)
Commercial API Small Molecules /Peptides Synthesis

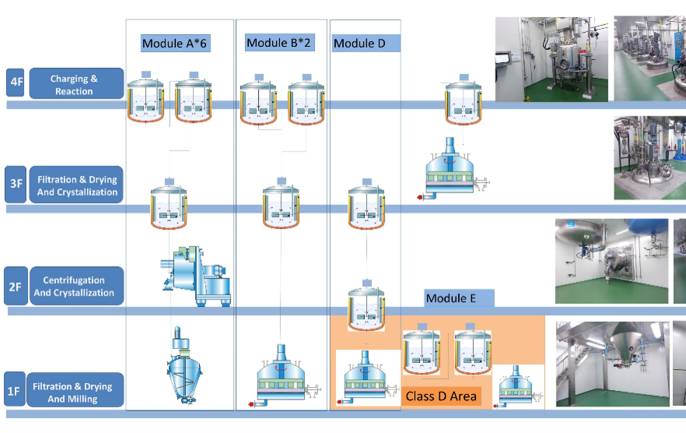
About the plant
The Shaoxing site, established in 2022 in China, received NMDA approval for commercial manufacturing in the same year and successfully completed a Pre-Approval Inspection (PAI) by the FDA in 2025.
Backed by a team of 3,500 process chemists, the site supports manufacturing activities from clinical to commercial scale for small molecules (APIs and advanced intermediates) as well as commercial peptides, utilizing reactors ranging from 5L to 12,500L.
A key strength of the site lies in its technological capabilities, including:
- Flow chemistry scaled to commercial production,
- High-potency chemistry (OEB-5) at commercial scale,
- Dedicated biocatalysis and chemical catalysis groups.
The site also houses a material sciences division capable of performing micronization, jet milling, wet milling, and spray drying for drug substances and APIs.
We operate segregated areas for:
- Non-GMP production, focused on customized building blocks and RSMs,
- GMP-compliant manufacturing of advanced intermediates and active pharmaceutical ingredients.
Additionally, the facility includes dedicated production areas for both human and veterinary APIs, ensuring compliance and specialization across product categories.
The site offers > 1,000,000 L capacity.
Compliance
- ISO
- FDA (cGMP)
- NMPA (China GMP)
Activity
- CHEMICAL-SYNTHETIC, RSM (Raw Starting Materials), Intermediates, Fine Chemicals, API (Active Pharmaceutical Ingredients), Building Blocks manufacturing
Features
- Uses: Investigational, Commercial (Phase IV), Preclinical, Phase I, Phase II, Phase III, Human, Veterinary
- Toxicity (OEB classification): 1 / low-hazard (PDE > 5,000 µg/day), 2 (PDE = 1,000 - 5,000 µg/day), 3 (PDE = 100 - 1,000 µg/day), 4 / HPAPI (PDE = 10 - 100 µg/day), 5 / HPAPI (PDE < 10 µg/day)
- Controlled substance: N/A
- BSL: N/A
- Therapeutic areas: (A) Digestive tract and metabolism, (B) Blood and blood forming organs, (C) Cardiovascular system, (D) Dermatologicals, (G) Genito urinary system and sex hormones, (H) Systemic hormonal preparations excl. sex hormones and insulins, (J) Antiinfectives for systemic use, (L) Antineoplastic and immunomodulating agents, (M) Musculoskeletal system, (N) Nervous system, (P) Antiparasitic products, insecticides and repellents, (R) Respiratory system, (S) Sensory organs, (V09-10) Radiopharmaceuticals, (V06) Nutrients, (V04) Diagnostic agents, (V01) Allergens
- Markets: FDA (USA), NMPA (China)
Batch Size / Reactor
- Small, Medium, Large
Services
- Regulatory services, Development services, Manufacturing services, Analytical / QC services, Quality Assurance services, R&D, QbD (Quality by Design), Process development, Process optimization, Process validation, Stability studies execution, ICH Stability studies, Tech transfer, Pilot plant, Engineering batches, Scale-up, Pilot batches, Registration batches, Batch records, Analytical methods development, Analytical methods validation, Release testing of raw materials, Analytical methods transfer, QP in house, Batch certification / release, GMP documentation, CMC regulatory support, IND (Investigational New Drug aplication) filing support, IMPD (Investigational Medicinal Product Dossier) submission support, NDA (New Drug Application) submission support, ANDA (Abbreviated New Drug Application) submission support, CTD/eCTD compilation and submission, DMF (Drug Master File) preparation and submission, Salt screening, Co-crystal screening, Polymorph screening, Crystallization screening, Chiral resolution screening, Milling, Safety studies, Solid form selection, Crystal engineering, PGI assessment (Genotoxic Impurities), Nitrosamine risk assessment, Customized block synthesis, Impurity synthesis
Address
No. 18, Jingqi East Road, Hangzhou Bay Shangyu Economic and Technological Development Area, Shangyu District, Shaoxing, Zhejiang, 312300, China
Year
2022
 China
China
Other plants of this company
Related information
Contact us
If you have any questions or suggestions, click here. We will be happy to assist you.






 Pharmaron API Commercial Synthesis Cramlington (UK)
Pharmaron API Commercial Synthesis Cramlington (UK) Pharmaron API Commercial Synthesis Coventry (US)
Pharmaron API Commercial Synthesis Coventry (US)