HIPRA BIOTECH SERVICES | Amer
End-to-end internal capabilities from cell line development to commercial manufacturing











About the plant
HIPRA Biotech Services is HIPRA’s CDMO division, bringing over 50 years of scientific and technical excellence to pharmaceutical and biotechnology companies, offering comprehensive contract development and manufacturing services in a wide range of biologics technologies and modalities.
- We have capacity available now to start development, clinical supply and commercial manufacturing projects in mammalian, microbial, and aseptic fill & finish.
- We are able to hit 12 months from Gene/Strain to Drug Substance as demonstrated by BIMERVAX® (which we adapt each year in 4-6 months) and our typical lead times demonstrated in dozens of programs in Animal Health
- We have been EMA approved for almost 30 years with an excellent inspection track record, and we are getting ready for FDA approval by mid-2026
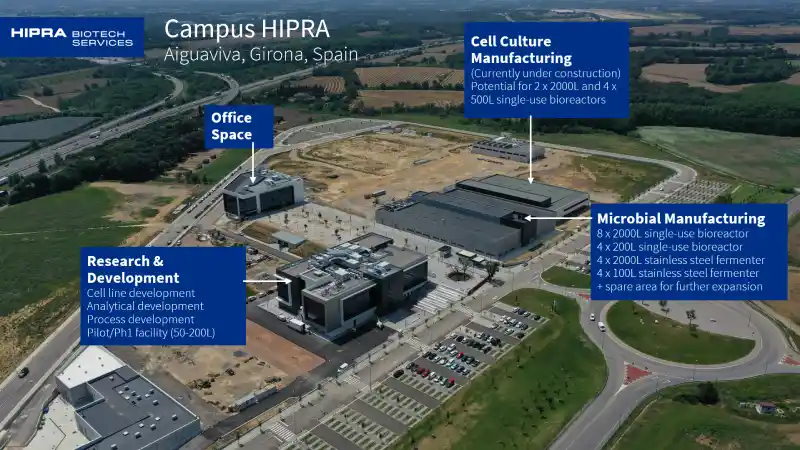
Our AIGUAVIVA campus features our R&D labs and our Microbial DS manufacturing expansion.
Our AMER campus offers our Microbial DS, Mammalian DS, Aseptic F&F, and QC services.
DEVELOPMENT (AIGUAVIVA campus)
From early discovery to clinical manufacturing production, we accelerate each stage of biologics drug development with expertise and flexibility.
Our AIGUAVIVA Development labs feature high-throughput bioreactor systems (Ambr®15 and Ambr®250) for design of experiments, small- and mid-scale benchtop bioreactors, Cobas® analyzers (for clinical chemistry and immunochemistry assays), and advanced tools for cell line development, complemented by a specialized area for process development and a dedicated GMP area for early-phase clinical batch production.
MANUFACTURING (AMER and AIGUAVIVA campuses)
We provide robust and flexible pharmaceutical manufacturing and quality control services at our state-of-the-art GMP certified facilities.
Our AMER DS facilities feature top-of-the-line single-use 500L cell culture bioreactors, 2000L microbial fermentation single-use bioreactors and stainless-steel fermenters, single-use chromatography, tangential flow filtration, and centrifuges.
Our AIGUAVIVA DS expansion facility features 2000L microbial fermentation single-use bioreactors and stainless-steel fermenters, single-use chromatography, tangential flow filtration, and centrifuges.
Our AMER DP facility features a high-speed isolator liquid vial filling line with 100% IPC.
Our AMER QC labs feature a wide variety of equipment enabling a wide range of methods; equipment is the same as used in the Analytical Methods development lab.
Compliance
- ISO
- EMA (EU GMP)
- FDA (cGMP)
- Health Canada (Canada GMP)
- World Health Organization (GMP / HACCP)
- ANVISA (Brazil B-GMP)
- ANMAT (Argentina nueva GMP)
- PMDA/MHLW (Japan GMP)
- ISP (Chile BPM)
- INVIMA
- MSPAS
- COFEPRIS
- DIGEMID
- MHRA (UK GMP)
- 9001
- 14001
Activity
- Abs (Antibodies), Peptide, protein, hormone & enzyme, Vaccines, Injectables, Fill&Finish, Small volume vials, FDF / DRUG PRODUCTS, BIOLOGICS manufacturing
Features
- Uses: Investigational, Commercial (Phase IV), Preclinical, Phase I, Phase II, Phase III, Human
- Toxicity (OEB classification): 1 / low-hazard (PDE > 5,000 µg/day), 2 (PDE = 1,000 - 5,000 µg/day), 3 (PDE = 100 - 1,000 µg/day)
- Controlled substance: N/A
- BSL: 1, 2
- Therapeutic areas: N/A
- Markets: DIGEMID (Peru), COFEPRIS (Mexico), INVIMA (Colombia), MSPAS (Guatemala), ISP (Chile), FDA (USA), EMA (EU), PMDA (Japan), Health Canada (Canada), MHRA (UK), ANVISA (Brazil), ANMAT (Argentina)
Batch Size / Reactor
- Small, Medium, Large, Batch, Fed-batch, 1 - 100 L, 100 - 1000 L, 1000 - 2000 L
Services
- Development services, Manufacturing services, Analytical / QC services, Quality Assurance services, Packaging, Logistics, Preformulation studies, R&D, Formulation / Galenic design, QbD (Quality by Design), Process development, Process optimization, Process validation, Stability studies design, Stability studies execution, ICH Stability studies, Comparability studies, Downstream, Fill&Finish, Cell line development, Cell banking, Sterile product manufacturing, Non sterile product manufacturing, Upstream, Tech transfer, Pilot plant, Formulation mixing, Research batches, Engineering batches, Scale-up, Pilot batches, Registration batches, Batch records, Analytical methods development, Analytical methods validation, Release testing of raw materials, Release testing of product, Bioanalysis, Analytical methods transfer, QP in house, Batch certification / release, GMP documentation, Primary packaging, Secondary packaging, Labeling, Serialization, Storage, Distribution, GDP
Address
Avinguda de la Selva, 135, 17170 Amer, Girona
Year
1971
 Spain
Spain
Documents
Other plants of this company
Contact us
If you have any questions or suggestions, click here. We will be happy to assist you.







 HIPRA BIOTECH SERVICES | Aiguaviva
HIPRA BIOTECH SERVICES | Aiguaviva