Pharmaron API Commercial Synthesis Coventry (US)
Commercial API Small Molecules Synthesis
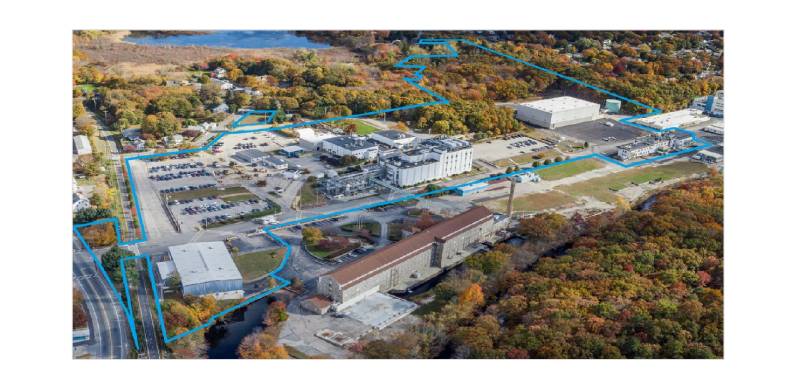
About the plant
The Pharmaron Coventry site, located in Rhode Island, USA, was acquired by Pharmaron in 2022, further strengthening the company’s technological capabilities and service offerings.
Coventry is an FDA-approved facility equipped with reactors ranging from 50 L to 8,000 L, enabling batch production from just a few grams to several hundred kilograms. The site supports the full development lifecycle—from clinical phases through to commercial manufacturing.
The facility is organized into a PR&D/pilot plant building and a commercial-scale production building, with a combined reactor capacity of 76,000 L.
Coventry stands out for its large-scale hydrogenation capabilities and is DEA-approved to manufacture controlled substances across Schedules I to V.
The site also plays a key role in Pharmaron’s successful hybrid China–US model: advanced intermediates are produced in China, shipped to Coventry, and converted into final APIs in the US. This model ensures a robust, flexible supply chain while maintaining cost-efficiency (COGs).
Compliance
- ISO
- FDA (cGMP)
- ECOVADIS
Activity
- CHEMICAL-SYNTHETIC, RSM (Raw Starting Materials), Intermediates, Fine Chemicals, API (Active Pharmaceutical Ingredients) manufacturing
Features
- Uses: Investigational, Commercial (Phase IV), Preclinical, Phase I, Phase II, Phase III, Human, Veterinary
- Toxicity (OEB classification): 1 / low-hazard (PDE > 5,000 µg/day), 2 (PDE = 1,000 - 5,000 µg/day), 3 (PDE = 100 - 1,000 µg/day), 4 / HPAPI (PDE = 10 - 100 µg/day)
- Controlled substance: High potential for abuse & no medical use, High potential for abuse & medical use, Lower potential for abuse, Low potential for abuse, Lowest potential for abuse
- BSL: N/A
- Therapeutic areas: (A) Digestive tract and metabolism, (B) Blood and blood forming organs, (C) Cardiovascular system, (D) Dermatologicals, (G) Genito urinary system and sex hormones, (H) Systemic hormonal preparations excl. sex hormones and insulins, (J) Antiinfectives for systemic use, (L) Antineoplastic and immunomodulating agents, (M) Musculoskeletal system, (N) Nervous system, (P) Antiparasitic products, insecticides and repellents, (R) Respiratory system, (S) Sensory organs, (V06) Nutrients, (V04) Diagnostic agents, (V01) Allergens
- Markets: FDA (USA), EMA (EU), PMDA (Japan), Health Canada (Canada), MHRA (UK), TGA (Australia), MFDS (South Korea)
Batch Size / Reactor
- Small, Medium, Large, 10-100 L, 100 - 1,000 L, 1,000 - 2,000 L, 2,000 - 5,000 L, 5,000 - 10,000 L
Services
- Development services, Manufacturing services, Process development, Process optimization, Process validation, Stability studies design, Stability studies execution, ICH Stability studies, Tech transfer, Pilot plant, Research batches, Engineering batches, Scale-up, Pilot batches, Registration batches, Batch records, Co-crystal screening, Crystallization screening, Chiral resolution screening, Milling, Crystal engineering, PGI assessment (Genotoxic Impurities), Nitrosamine risk assessment
Address
498 Washington Street, Coventry, Rhode Island, 02816, United States
Year
1991
 China
China
Other plants of this company
Related information
Contact us
If you have any questions or suggestions, click here. We will be happy to assist you.






 Pharmaron API Commercial Synthesis Cramlington (UK)
Pharmaron API Commercial Synthesis Cramlington (UK) Pharmaron API Commercial Synthesis Shaoxing (China)
Pharmaron API Commercial Synthesis Shaoxing (China)