
2026-02-12
Beyond Capacity: How Smarter Science is Redefining Biologics Manufacturing
See more
2026-02-04
What truly matters when selecting a Contract Manufacturing Organization for clinical and commercial success
See more
2026-01-30
Regulatory expertise can be the difference between a smooth veterinary drug approval and costly delays.
See more
2026-01-07
Pharmaron is a leading life sciences service provider, offering a wide range of research, development, and manufacturing capabilities across the entire drug discovery, preclinical, clinical development, and commercialization process.
See more
2025-11-05
Mabion is a CDMO company specializing in the advanced manufacturing and development of biological products, with extensive experience in monoclonal antibodies. Founded in 2007 and headquartered in the EU, in Poland.
See more
2025-10-15
Cultiply is a specialized biotechnology company dedicated to industrial microbiology and fermentation technologies for biological processes. This company is focused on offering a complete innovation platform, from the idea phase to industrial scaling.
See more
2025-10-01
How In-licensing and Out-licensing Drive Growth, Collaboration, and Speed in Drug Development
See more
2025-08-22
Connecting Leaders in Biologics Manufacturing
See more
2025-06-20
MAI CDMO is now available in 5 languages for a seamless multilingual experience.
See more

2025-04-30
Practical SEO strategies for CDMOs to increase online visibility and attract pharma and biotech partners.
See more
2025-04-14
Discover How CMOs and CDMOs Drive Innovation, Scale, and Speed in Drug Development
See more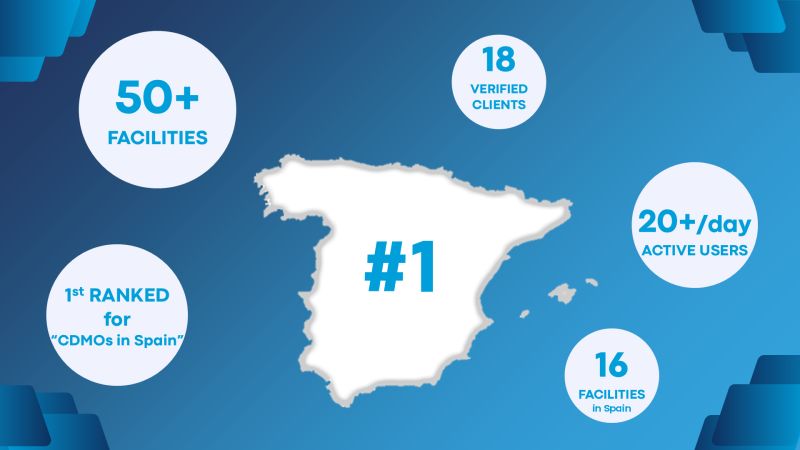
2025-03-20
MAI CDMO continues to revolutionize the pharmaceutical outsourcing industry,
See more
2025-03-10
Expanding Partnerships in the Nutraceutical Industry
See more

2025-02-05
MAI CDMO earns Startup certification under Spain’s Law 28/2022, fostering innovation.
See more
2025-01-30
Join Us at Pharma Contract Manufacturing 2025 – Let’s Shape the Future of Pharma Together!
See more
2024-12-30
Empowering Innovation: Celebrating 10 Years of Success at BIC Bizkaia
See more
2024-11-13
Strengthening Our Technological Backbone: Jon Barandiaran Joins MAI CDMO to Enhance IT Security and Innovation
See more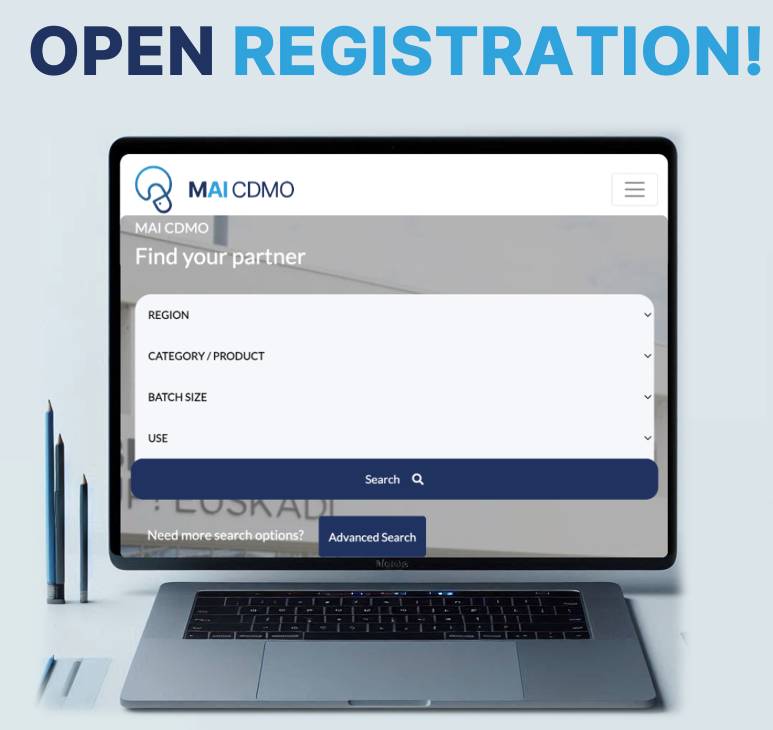
2024-11-05
Why MAI-CDMO is the Ultimate Platform to Streamline Your Search for Trusted Contract Manufacturing Partners
See more
2024-10-23
Boosting Innovation and Growth for Startups
See more
2024-10-20
MAI CDMO Strengthens Industry Ties and Showcases Innovation at CPHI Milan, Paving the Way for Future Collaborations
See more
2024-10-06
MAI CDMO Welcomes Juan Basterra, CEO of Mikrobiomik, as New Advisor.
See more
2024-09-17
MAI CDMO to Unveil New Service at CPhI Milan: Transforming the CMO and CDMO Industry
See more
2024-09-16
MAI CDMO Embarks on a New Chapter in Pharma Connections
See moreWelcome to MAI CDMO’s Industry News & Insights
At MAI CDMO, we understand that staying informed on the latest developments in the pharmaceutical and biotechnological sectors is crucial. Our Industry News & Insights section provides the latest updates, trends, and best practices related to contract development and manufacturing organizations (CDMOs), focusing on how these advancements are transforming pharmaceutical outsourcing. Here, you’ll find insights into key areas like drug development, clinical trials, advanced therapeutic manufacturing, regulatory updates, and more.
Why Industry News Matters
In the pharmaceutical and biotech industries, continuous innovation and adaptation are critical. The field of contract development and manufacturing is rapidly evolving, and staying up-to-date on these changes is essential for companies looking to optimize their drug development timelines, control manufacturing costs, and ensure compliance with global regulatory standards. Our Industry News section is your go-to resource for understanding shifts in CDMO services, emerging technologies, and regulatory frameworks.
Explore Current Trends in Pharmaceutical Outsourcing
Growing Demand for Advanced Therapies
With the rise of gene and cell therapies, CDMOs are increasingly focusing on manufacturing solutions for advanced therapeutic medicinal products (ATMPs). These cutting-edge therapies require specialized facilities, skills, and regulatory know-how to meet stringent standards. As a result, more CDMOs are investing in technologies and expertise to support ATMPs, allowing pharmaceutical companies to bring innovative treatments to patients faster and more efficiently.
Shift Toward Flexible Manufacturing
Flexibility in manufacturing is becoming a defining feature for CDMOs. Pharmaceutical companies need the ability to scale production up or down, switch between small and large batch sizes, and adapt processes for various drug forms, from oral solids to injectables. This flexibility not only improves efficiency but also reduces time-to-market for new drugs, an essential factor in today’s competitive landscape.
Increasing Importance of Digitalization and Automation
CDMOs are adopting advanced digital and automation technologies to streamline processes, improve accuracy, and enhance productivity. These technologies are especially crucial for meeting quality and regulatory standards, as they reduce human error and provide real-time data tracking. For clients, these advancements mean a more transparent and efficient outsourcing process, which translates to cost savings and faster development timelines.
Regulatory Changes and Compliance Standards
The global regulatory environment is always evolving. CDMOs must stay informed about regulatory changes from agencies like the FDA, EMA, and other global authorities. This section includes updates on significant regulatory changes, such as those impacting clinical trial protocols, data management, and safety standards. Staying ahead of regulatory updates ensures that CDMOs can maintain compliance and continue to meet the highest quality standards, which is vital for their clients` success.
Sustainable and Green Manufacturing Practices
Sustainability is a growing concern across industries, and pharmaceuticals are no exception. Many CDMOs are adopting green chemistry practices, waste reduction initiatives, and energy-efficient processes to reduce environmental impact. By prioritizing eco-friendly manufacturing, CDMOs are not only addressing regulatory pressures but also aligning with the values of environmentally conscious clients and investors.
Case Studies: Success Stories in Pharmaceutical Outsourcing
Our Industry News section also includes success stories showcasing how MAI CDMO clients have leveraged outsourcing to overcome challenges and accelerate their projects. From small biotech startups to established pharmaceutical firms, learn how companies have used MAI CDMO’s platform to find specialized CDMO partners, access advanced manufacturing capabilities, and achieve significant milestones.
Exclusive Resources for MAI CDMO Clients
At MAI CDMO, we’re committed to empowering our clients with more than just a directory. Our Industry News section offers:
Stay Ahead with MAI CDMO
The pharmaceutical industry’s landscape is constantly shifting, and being informed is your key to staying competitive. Our Industry News section is regularly updated with new content, so you can rely on MAI CDMO for the latest in CDMO developments and pharmaceutical outsourcing. Whether you`re in early-stage research, clinical trials, or commercial manufacturing, we provide the insights you need to make informed decisions, mitigate risks, and optimize your outsourcing strategy.
Explore MAI CDMO’s Industry News & Insights section regularly to stay connected with the trends that matter most in drug manufacturing, development, and regulatory compliance. And if you need help finding the right CDMO partner for your project, MAI CDMO’s platform is here to simplify your search, save time, and ensure quality connections.
If you have any questions or suggestions, click here. We will be happy to assist you.





